
Coupling with gas chromatography was performed by C. Field applied chemical ionisation in 1966, which was followed by the ‘thermospray’ method, introduced by the C.R. Stephens assembled a time-of-flight (TOF) device, and, in 1953–1958, W. Milligan introduced the method of ionisation and pass through a magnetic sector. The first mass spectral measurements were undertaken by J.J. The first MS stage serves for selection of an ion, whereas the second analyses of ions result from fragmentation of the ion separated in the first stage. There are also devices (the MS-MS tandem) allowing for analysis of mixtures without prior chromatography. The devices may be either used with a system for direct sample introduction (pure substances) or coupled with a chromatographic system.
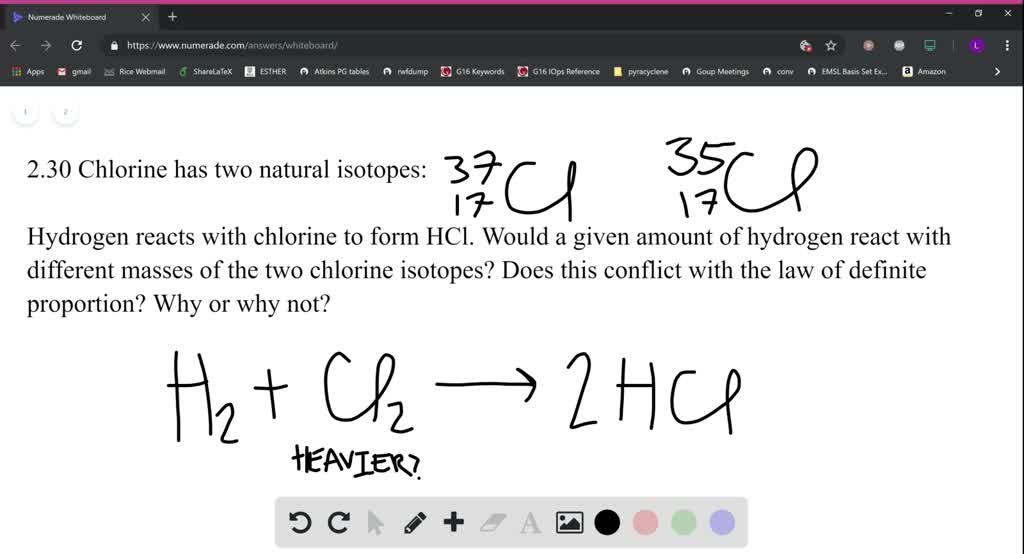
Traditionally limited to the study of classical small organic molecules (M 100,000), polypeptides in particular. The detector measuring the relative abundance of each ion The dispersion system, the analyser, which ensures ion separation by mass/charge ratio The source where ionisation of molecules and ion fragmentation occurs The chapter presented here is an introduction to mass spectrometry, which, we think, helps the understanding of the mechanism of fragmentation corroborating spectral data and molecular structures. At the end of the chapter, acquisition of mass spectrum is discussed. Fragmentation schemes are followed by the simplified spectra, which help the understanding of such complex phenomena.
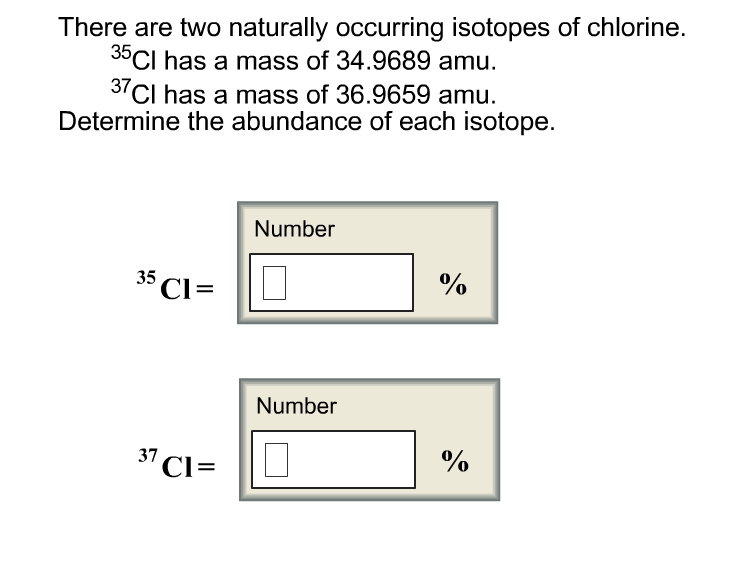
In the second part of the chapter, the molecular fragmentation for alkanes, iso-alkanes, cycloalkanes, halogen, alcohols, phenols, ethers, carbonyl compounds, carboxylic acids and functional derivatives, nitrogen compounds (amines, nitro compounds), sulphur compounds, heterocycles and biomolecules (amino acids, steroids, triglycerides) is explained. A frame time of the main theoretical milestones in both theory and experimental mass spectrometry is highlighted here. A paragraph emphasises the ionisation energy issues, the basics of ionisation voltage, the developing potential and the energy balance. Isotopic percentage and nominal mass calculation are also explained along with fragmentation mechanism. First, the fundamental notions of mass spectrometry are explained, so that the reader can easily cover this chapter (graphs, main pick, molecular ion, illogical pick, nitrogen rule, etc.).
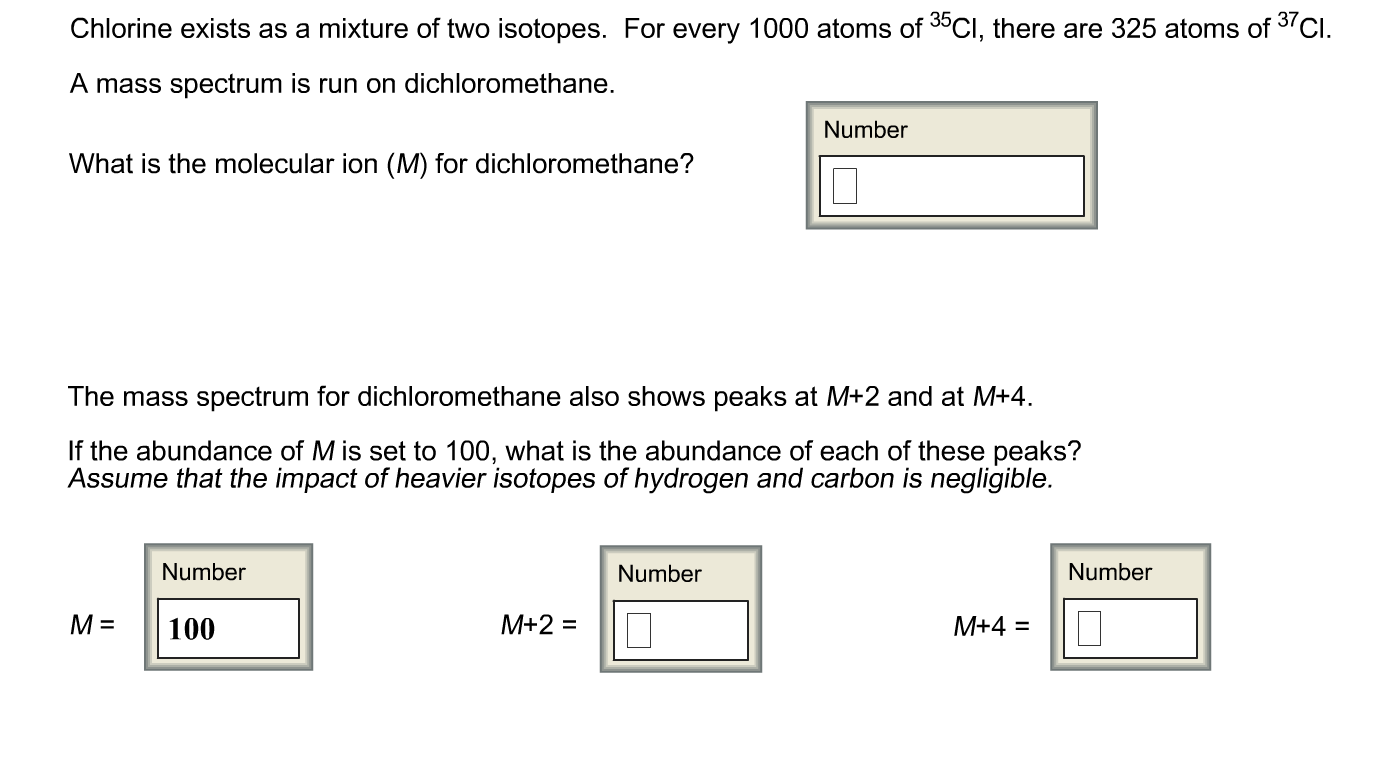
The chapter includes an introduction to the main ionisation techniques in mass spectrometry and the way the resulting fragments can be analysed.


 0 kommentar(er)
0 kommentar(er)
